Homepage
Translating color & fluorescence to biomarker concentrations
Essential tools for making the right patient care decisions.
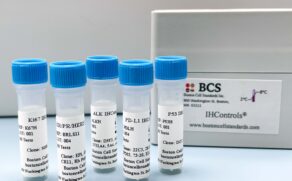
Quantitative IHC Standards:
What they are & why they’re important

Technologies that seemed fine at the time…




Providing IHC laboratories technology for the age of precision medicine
Featured in the Press
Highlighted by Editorials
We Deliver the Tools to Enable Best Practices in IHC
The only immunohistochemistry calibrators. Period.
The only liquid suspension IHC controls, delivering unparalleled homogeneity, reproducibility, and low-cost.
Innovative products delivering essential IHC performance metrics.
*Covered by patents EP3514543B1, EP3065704B1, and others pending.